Kinga Kowalewska-Grochowska
University of Alberta, Department of Medical Microbiology and Immunology,
APL Provincial Laboratory for Public Health, Edmonton AB
Romina C Reyes
University of British Columbia, Department of Pathology and Laboratory Medicine, Vancouver, BC
LifeLabs, Burnaby, BC
Pauline Tomlin
University of Alberta Department of Laboratory Medicine and Pathology
APL Provincial Laboratory for Public Health, Edmonton AB
Diagnostic parasitology has been affected by many recent changes – not only changing patient populations, but also shifts in workforce. Globalization, human/animal migration and climate change influence epidemiology of parasitic diseases and broaden their distribution. Previously considered unusual parasites are now becoming a part of mainstream health concerns.
To make matters worse, the pool of people with specialized diagnostic knowledge and expertise is shrinking due to retirements and burnout, without adequate replacement through incoming workforce. This creates additional strain on the specialty and requires immediate action to address the problem, as it becomes progressively worse. It also requires rethinking of our older approaches and a complete paradigm shift.
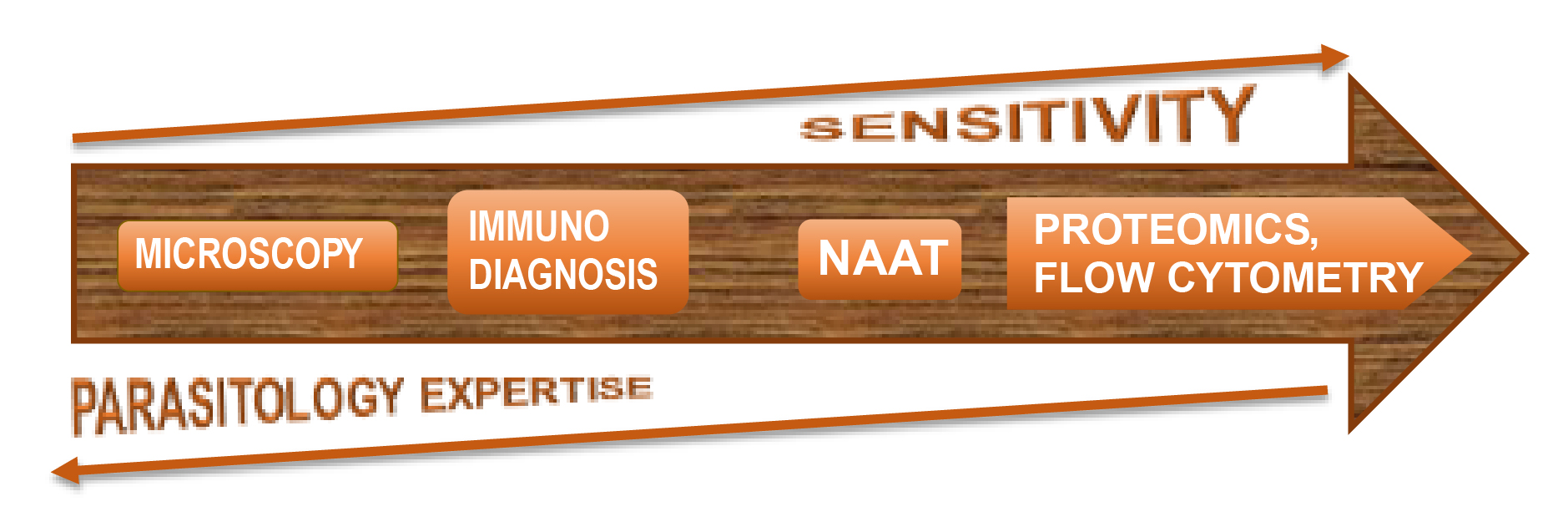
For years, microscopy was the diagnostic gold standard. It included examination of wet mounts, supplemented by stains (iron hematoxylin or trichrome for stools, or Wright and Giemsa for blood and tissue parasites), with later addition of targeted Ziehl Neelsen and Kinyoun stains for acid fast organisms, as well as simpler non-specific fluorescent stains, like acridine orange or calcofluor white. Unfortunately, no matter how cheap and accessible these stains are: microscopy is labour and time intensive and requires expertise and access to appropriate biological material.
Serology-based assays for parasite antigens and antibodies became the next step in diagnostic evolution. These tests were invaluable in situations where experienced microscopists – and representative tissues or fluids – were not available. Popular methodologies included enzyme-linked immunosorbent assay (ELISA), indirect or direct immunofluorescent antibody (IFA or DFA) assays, hemagglutination (HA) or complement fixation (CF) tests, as well as immunoblotting, followed later by newer techniques such as luciferase immunoprecipitation system (LIPS), and many others.
In the end, it was the rise of molecular biology and its foray into diagnostics that changed the landscape of parasitology forever. Many challenges of microscopy and serology could now be overcome since nucleic acid amplification tests (NAAT) offered much greater sensitivity and specificity. Traditional PCR (including nested PCR) was soon followed by the real-time PCR (RT-PCR) for many parasites, with multiplexed PCRs able to pick up multiple sequences in the same reaction tube and thus diagnose multiple infections simultaneously. Many newer technologies emerged in short succession such as loop-mediated isothermal amplification (LAMP) and Luminex-based assays, along with the promising newcomers: proteomics (e.g. mass spectrometry) and flow cytometry.
Diagnostic parasitology, however, doesn’t only rely on new technologies – but also on new approaches. One such example is syndromic panel testing, currently changing the way microbiology laboratories address the diagnoses of infectious diseases. Syndromic molecular panels allow for focused and integrated workflows replacing routine culture and microscopy work that relied on varied and diverse technical expertise. These panels are able to include and detect a variety of viral, bacterial, fungal and even parasitic pathogens; they are focused on a single clinical syndrome such as gastrointestinal infections. While the molecular targets on most multiplex GI (gastrointestinal) panels (FDA cleared) can include an extensive list of bacterial targets – the parasitic ones are limited and often include only Giardia lamblia, Cryptosporidium spp, Entamoeba histolytica and Cyclospora cayetanensis. As a screen, these panels work well to cover the most common agents of parasitic GI infections. However, they do not detect worms, eggs, or any other parasitic species that are also clinically important and highly prevalent in certain populations.
In January 2023 the British Columbia Ministry of Health under the Laboratory Services Act approved a pathogen multiplex panel fee code item to encourage diagnostic microbiology laboratories to move towards syndromic testing. This will have a significant positive impact by increasing detection of those parasites that are included in the molecular target menus, shortening turnaround times leading to faster diagnosis and better management of most common parasitic infections. At the same time, however, this approach will decrease, or delay detection of ova and parasites routinely picked up by the traditional microscopy. Therefore, laboratories will have to work diligently to educate healthcare providers of the limitations as well as benefits of syndromic testing.
As enticing as it may be, in general all non-morphology-based tests come with limitations. First and foremost, these tests (syndromic panels or individual NAATs or proteomics) do not cover all medically important parasites. Humans can harbor at least 848 species of parasites – of which about 90 are common. NAATs only cover a few of these since current sequence reference databases are still inadequate. Both serology and PCR do not distinguish active from past infections due to persistence of antibodies or parasite DNA.
Finally, morphological description is still considered essential for detection and naming of newly discovered parasite taxa.
The broad universality of microscopy contrasts starkly with targeted nature of NAATs. Because of that, molecular methods should complement rather than replace morphological assessment in many settings – much as, in bacteriology, Gram stain still coexists with next generation sequencing for almost 140 years.
All things considered the increased reliance on molecular detection methodology is a double-edged sword: on one side, it saves time (if not money) and addresses the shortage of specialized workforce, but on the other hand, it creates progressive, widespread loss of skills and morphology expertise for parasite identification. This in turn has potential to negatively impact patient care – and, in a vicious circle, affects our ability to carry out further diagnostic research, or train artificial intelligence (AI) to replace inevitable loss of human expertise. As such, we must find a way to support and preserve both.
References:
Dien Bard J, McElvania E. Panels and Syndromic Testing in Clinical Microbiology. Clin Lab Med. 2020 Dec;40(4):393-420. doi: 10.1016/j.cll.2020.08.001. Epub 2020 Oct 1. PMID: 33121611; PMCID: PMC7528880.
http://www.bccss.org/bcaplm-site/Documents/Health%20Professionals/ROD%20Summary%20Payment%20Schedule.pdf
Bradbury RS, Sapp SGH, Potters I, Mathison BA, Frean J, Mewara A, Sheorey H, Tamarozzi F, Couturier MR, Chiodini P, Pritt B. Where Have All the Diagnostic Morphological Parasitologists Gone? J Clin Microbiol. 2022 Nov 16;60(11):e0098622. doi: 10.1128/jcm.00986-22. Epub 2022 Oct 31. PMID: 36314793; PMCID: PMC9667774.
Ramanan P, Bryson AL, Binnicker MJ, Pritt BS, Patel R. Syndromic Panel-Based Testing in Clinical Microbiology. Clin Microbiol Rev. 2017 Nov 15;31(1):e00024-17. doi: 10.1128/CMR.00024-17. PMID: 29142077; PMCID: PMC5740973.
Ndao M. Diagnosis of parasitic diseases: old and new approaches. Interdiscip Perspect Infect Dis. 2009;2009:278246. doi: 10.1155/2009/278246. Epub 2009 Dec 30. PMID: 20069111; PMCID: PMC2804041
Momčilović S, Cantacessi C, Arsić-Arsenijević V, Otranto D, Tasić-Otašević S. Rapid diagnosis of parasitic diseases: current scenario and future needs. Clin Microbiol Infect. 2019 Mar;25(3):290-309. doi: 10.1016/j.cmi.2018.04.028. Epub 2018 May 3. PMID: 29730224.



