By Selvarani Vimalanathan
Part 1: Influenza virus A
Background
Respiratory infections caused by influenza viruses (Influenza A and B) or respiratory syncytial viruses (RSV) can be mild to severe depending on the risk group. Healthy adults and children are usually able to recover from symptoms within a week or two. However, high risk groups including infants, the elderly, and immunocompromised adults, can develop severe illness after infection with influenza or RSV viruses which can lead to pneumonia, consequent hospital admission, and even death.
Each year, the flu epidemic is caused by two types of influenza viruses: Influenza A subtypes (H3N2 and H1N1) and influenza B; moreover, both influenza A and B viruses undergo mutations, with influenza A undergoing mutation more rapidly than influenza B. This makes the antibodies produced by infection or vaccine have partial or no protection against other virus type or subtype.
Objectives
This year, in collaboration with BC Centre for Disease Control (BCCDC), CMPT developed and established the respiratory virus proficiency testing panel customized to provide PT samples to various testing sites including point of care and hospitals. CMPT respiratory panel (RP) comprised of current circulating isolates of viral strains obtained from BCCDC which include influenza virus A subtypes (H3N2, H1N1), Influenza B and RSV subtypes RSV A and RSV B.
Viruses for the Respiratory PT Panel
The samples acquired from were amplified and identified. Each PT panel included three samples to be tested for influenza A (subtype H3N2 or H1N1), three samples to be tested for influenza B, and three samples to be tested for RSV (subtype A or B).
Virus propagation, inactivation and preparation for RP
Influenza type A subtypes H3N2 and H1N1, Influenza B, and RSV types A and B positive specimen were obtained from BCCDC and propagated in appropriate cell lines, clarified, quantified, and inactivated. Viral titer was measured using either plaque assay or TCID50 (Tissue culture infectious dose of 50%) method. The concentration virus ranged from 6×10^6 to 1.5×10^7. Virus stocks were inactivated using an inactivation method provided by BCCDC. Effective inactivation, was tested using plaque assay or TCID50 assay
Stability Testing of Influenza A PT samples
In order to select the dilutions to be used as PT samples, the stock preparations were diluted 1/10, 1/100, and 1/1000, in viral transport media and 1mL of each dilution was aliquoted into several sterile O ring tubes. The sample stability tests were carried out to mimic the shipping conditions (room temperature) and also storage conditions (4°C) after shipping. To evaluate the above mentioned condition, samples were left at RT and at 4 C and the rate of deterioration was evaluated for a period of 4 weeks at different time points. Samples were taken on day 0 to represent shipping day, and tested after 7, 14, 21 and 28 days. The stability was confirmed by both antigen (BD) (Fig 1) and nucleic acid methods. Results obtained from antigen and nucleic acid method are shown in Fig 1 and Table 1. To avoid cross contamination, each virus was prepared at different time or day.
H3N2 BCCDC strain stability test
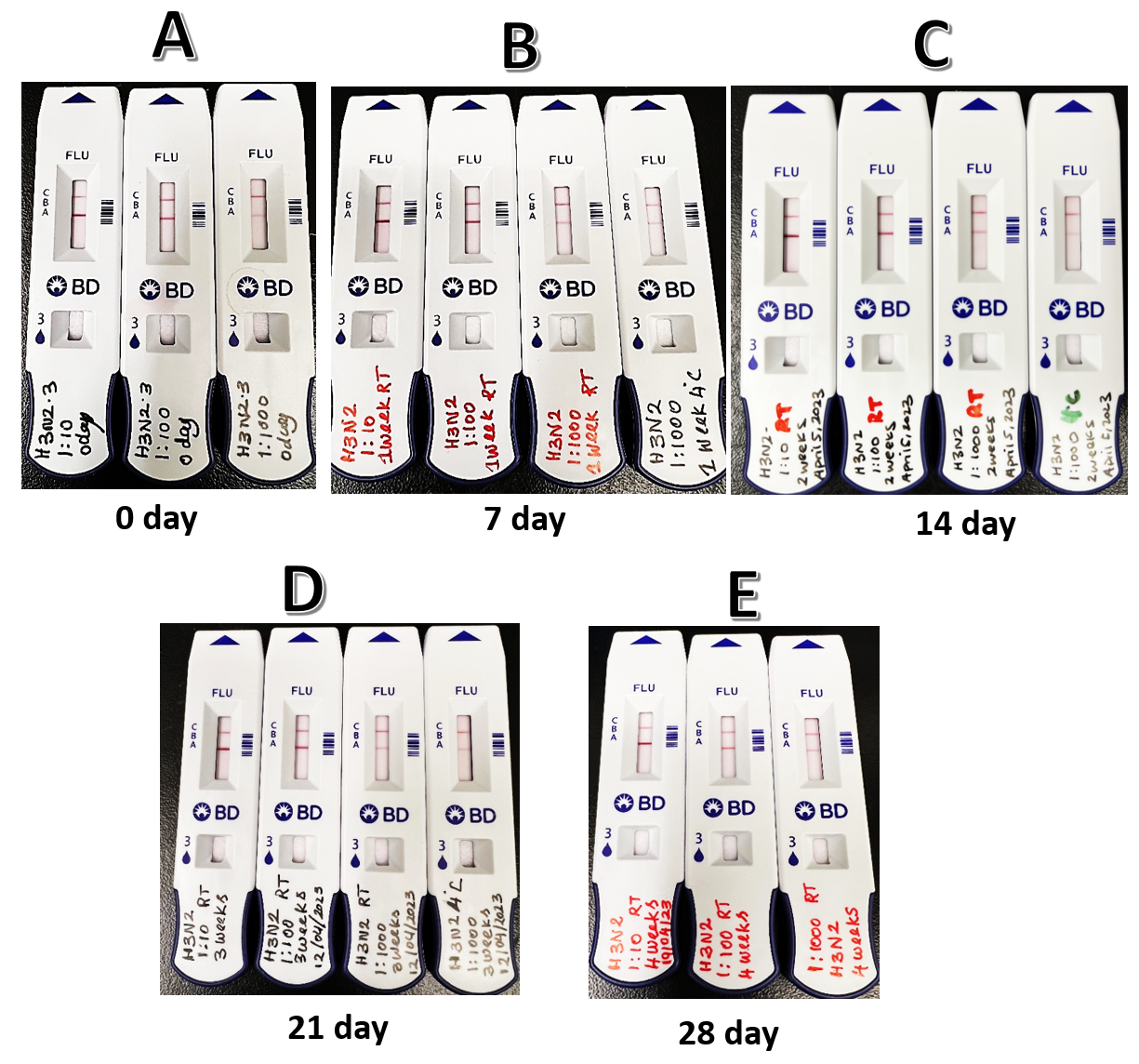
Figure 1. Testing results for H3N2 samples using the BD antigen test kit A: on Day 0; B: day 7; C: day 14; D: day 21 E: day 28
| Virus Type | No of days at RT | Subtype | Dilution | |
| Ct VALUES | ||||
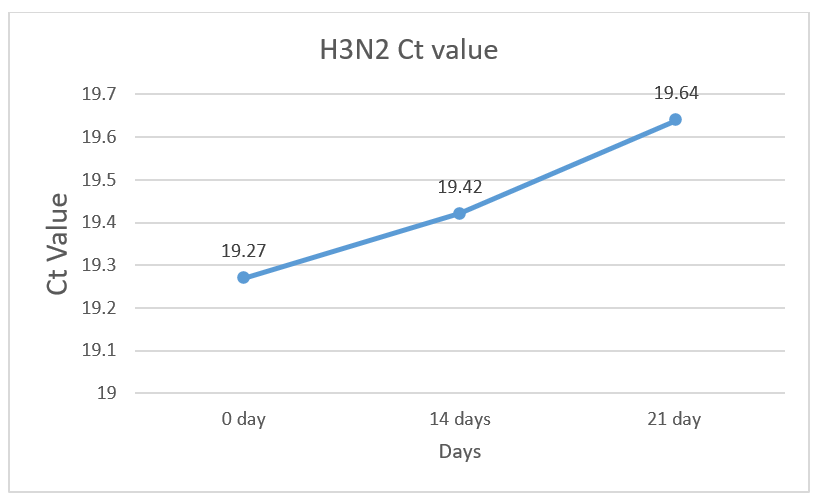
| Influenza A | 0 | H3N2 | 1/100 | 19.27 |
| Influenza A | 14 | H3N2 | 1/100 | 19.42 |
| Influenza A | 21 | H3N2 | 1/100 | 19.64 |
Table 1: Ct values of Influenza A H3N2 positive samples (1/100 dilution) measured at different time points.
Stability Test Results
Although a slight increase was noticed in the Ct values with time, the values at day 14 and day 21 remained quite close to the original at day 0.
The Influenza A dilutions showed good stability at RT after 21-28 days which is optimal for the development of PT samples that are shipped across the country.
CMPT launched the Respiratory Panel PT program in April 2023 to provide testing laboratories with an EQA tool to verify their performances and satisfy accreditation requirements.
EQA programs like this are an integral element of strong laboratory and health systems because they provide an objective service of external assessment in accordance with international standards of quality.
For more information on this PT program please contact CMPT.
Part 2 of this article will be published here, and announced in our newsletter. Please subscribe for updates.



