Early in 2021, CMPT in collaboration with BC Centre for Disease Control (BCCDC) created a COVID-19 proficiency testing program geared to point of care (POC) testing sites within the different Health Authorities. As testing became widespread and rapid testing systems began to proliferate, the College of Physicians and Surgeons of British Columbia, Diagnostic Accreditation Program (DAP), required all testing sites – private and public- to be accredited.
Participating in a COVID-19 proficiency testing (PT) program is one of the requirements for this accreditation.
The annual PT program consists of 6 shipments of 1 set of 4 samples that are either positive or negative for Covid-19 antigen and RNA. The first shipment was sent on March 2021 and the last one for the year, on January 2022.
We present here a year worth of experience and data.
Participants
The program started with mostly POC testing sites belonging to the different BC Health Authorities.
As the testing demand grew for different industries and travel, we saw a steep increase in private laboratories joining our program during the year (Figure 1).
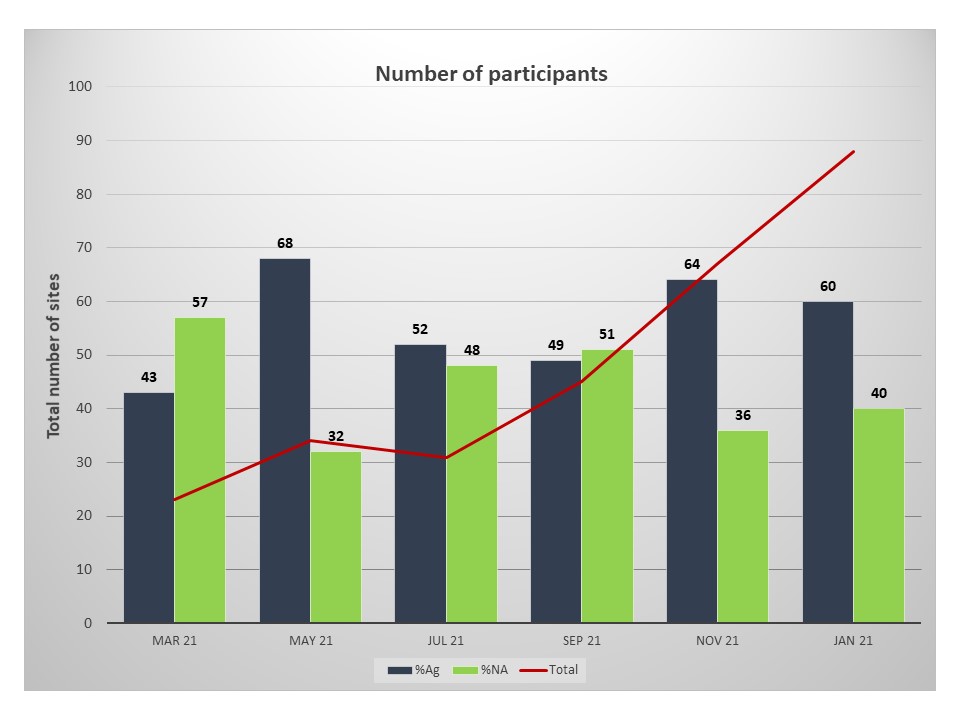
Figure 1. Number of Covid-19 PT participants. Percentage of participants performing Rapid Antigen tests (blue) and Nucleic Acid detection tests (green).
Participants used both Antigen and Nucleic acid detection methods evenly during the year with an increasing trend towards the use of Antigen tests towards the end of the PT year.
As presented in our Summer 2021 issue of Connections, CMPT adopted a plan to send samples with different concentrations of the viral material to better represent clinical samples. Two concentrations were chosen with the strongest being a 1:10 dilution and the second a 1:50 dilution (referred to as medium positive) of the original inactivated virus material provided by BCCDC.
We observed a big difference in the performance between nucleic acid detection methods and antigen detection methods, with the rapid antigen tests significantly lagging behind. CMPT investigated possible causes for this sensitivity difference between the methods and we found that testing sites were having difficulty getting positive results with the medium positive samples (Figure 2).
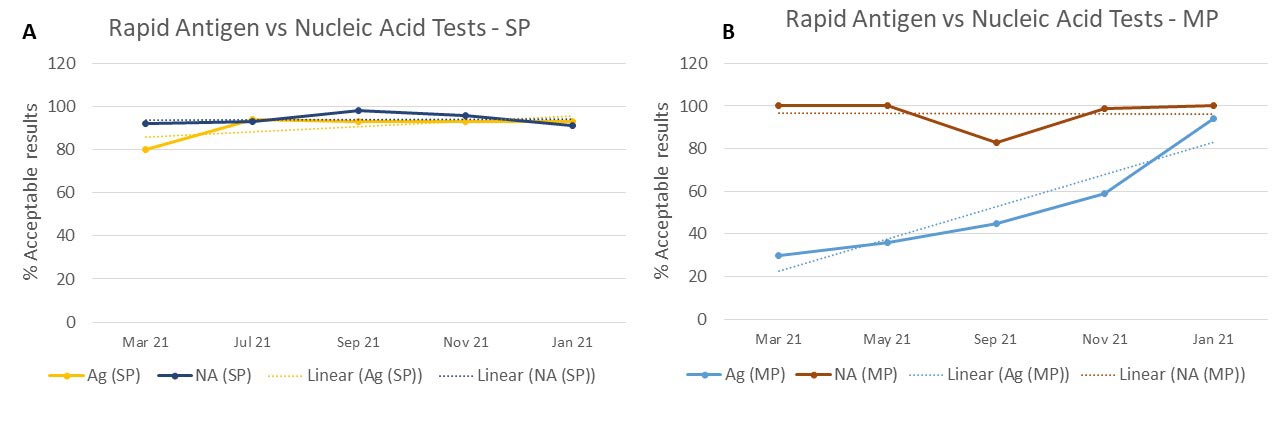
Figure 2. Performance of Rapid Antigen and Nucleic Acid tests A) when testing strong positive samples; B) when testing medium positive samples. Percentage of acceptable results
One of the issues found was insufficient training of the people that were performing the testing; this was addressed by the Health Authorities through in person re-training and by CMPT through the creation of a training video specifically geared to the process of CMPT PT samples.
With time, laboratories got better at detecting the medium positive sample (Figure 3) and thus, the performance of sites using Antigen methods improved and reached almost the same % of acceptable results than those for the Nucleic acid methods. We believe this was the result of gained experience and ongoing training efforts.
Figure 3. Performance of Covid-19 testing laboratories over time. Percentage of acceptable results.
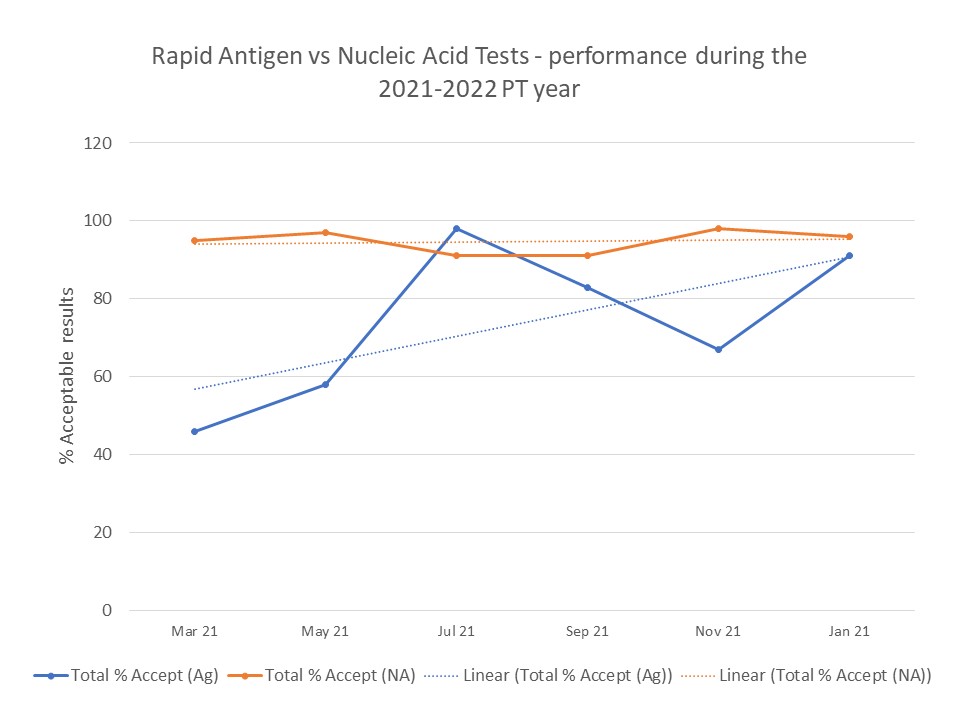
Overall, the Covid-19 PT program has been very successful and CMPT has been able to assist with the needs of a quite different client group. Provincial testing requirements were changing quickly and the different industries had to adapt to provide adequate testing options.
Entering now into our second year of Covid-19 PT we believe that CMPT has played a key role in enabling laboratories to offer Covid-19 testing that is reliable and fit for purpose.
Veronica Restelli, Editor



