CMPT has been examining the extra analytical phases for a long time. In 1997, CMPT took a major departure from the traditional PT testing by making clinical relevancy of reports its primary target, focusing on the interpretation, clarity, and appropriateness of laboratories’ reports.
Samples and challenges are designed to ensure laboratories report normal flora or contamination as such, and that the final report is clinically relevant. Challenges can also be designed to check laboratories’ adherence to current guidelines or appropriateness of susceptibility results reporting.
Challenge M091-5 consisted of gram negative bacillus in a CSF. Although laboratories reported the right susceptibility result interpretations, 31% of the laboratories reported antimicrobial agents that are specifically not recommended for treatment of CSF infections. This was addressed in the evaluation of results and in the educational critique.
Challenge M114-3 (2012) was a simulated vaginal screen sample with group B Streptococcus. The purpose of the challenge was to evaluate the adherence to the 2010 CDC guidelines on prevention of group B streptococcal disease in neonates which recommended against reporting erythromycin results. Sixty per cent of laboratories reported erythromycin results, making it a good educational opportunity for the participant laboratories.
In 1998-1999, CMPT introduced the Paper Challenge (PC) as a PT tool to further evaluate the extra-analytical phase. These challenges are created through a process that involves the selection of a topic, the description of a scenario, the design of possible answers, and the selection of the best response and unacceptable ones. Group analytics is applied and an informative critique with results and inter-laboratory comparison is written. The whole process is carried out by the CMPT’s Advisory Committee.
This year, CMPT introduced a new tool, the Video Challenge (VC) which uses a ~1.5 min video clip to present a scenario. The VC has the advantage of being able to present more complex scenarios and that the error is not so obviously stated as in the PC.
Figure 2. Distribution of PC/VC sent and reported errors.
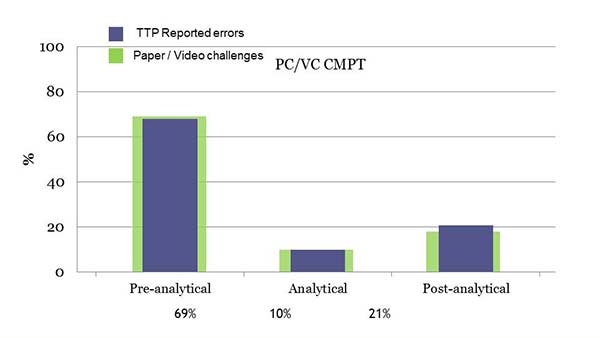
Since the beginning of the PC/VC program, CMPT sent 39 challenges, 69% targeting the pre-analytical phase, 10% targeting the analytical phase, 18% targeting the post-analytical phase, and 1 challenge related to safety issues. This distribution matches the error distribution observed in the literature (Figure 2).
In summary, it is important to extend the EQA to cover areas rarely challenged by PT schemes and where most of the errors tend to occur. The approaches may be “less technical,” but there is room for developing creative ways to address those areas.
Laboratory professionals, EQA providers, and accreditation bodies must increase their efforts to ensure quality is extended throughout the total laboratory testing process.
Suggested readings
Kristensen et al. How to conduct External Quality Assessment Schemes for the pre-analytical phase? Biochemia Medica 2014;24(1):114-22
Plebani M. Performance specifications for the extra-analytical phases of laboratory testing: Why and how. Clin. Biochem. 2017
Hawkins R. Managing the Pre– and Post-analytical Phases of the Total Testing Process. Ann. Lab. Med 2012. 32:5-16



