
by Veronica Restelli
EQA is an essential component for assuring quality testing in a laboratory. By participating, laboratories gain confidence in the quality of their performance, can evaluate staff competency, compare their performance with other laboratories, and take advantage of educational opportunities.
In recent years, the increasing interest in quality improvement and patient safety has made laboratories increasingly aware than focusing only in the analytical phase is not enough, and that the assurance of quality has to be extended throughout the total testing process.
Unfortunately, many EQA providers do not systematically assess the pre- and post-analytical phases, focusing instead only on the analytical quality. This approach is outdated since laboratory testing is increasingly reliant on analytical instruments, thereby making EQA schemes directed to the analytical phase a measurement of equipment and manufacturer’s performance rather than of laboratory performance.
Most EQA schemes do not tend to look at how laboratories handle improper containers or transport, compromised samples, mislabeled samples and neither do they address the appropriateness of the final report. Are the negative samples or contaminated reported as such? Are complex samples referred? Are the reports reaching the right patient or physician?
Evidence collected in the last few decades demonstrates that pre- and post-analytical phases of the total testing process (TTP) are more prone to error than the analytical phase. Figure 1 represents the different phases of the TTP, with 90% of the reported errors falling within the pre- and post-analytical phases, and the analytical phase accounting only for 10%. The problem with EQA targeting the analytical phase is then evident: 90% of the errors remaining unchallenged.
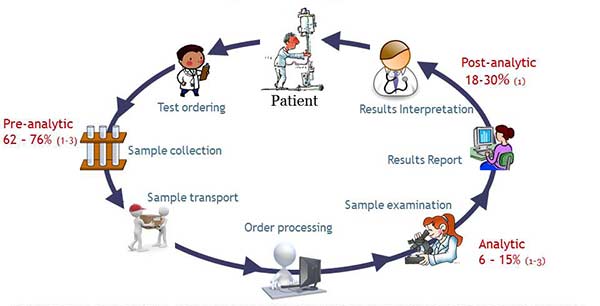
Figure 1. Total Laboratory Testing Process. In red: percentage of errors reported in that phase according to: (1) Restelli V., Taylor A., Cochrane D., Noble M. (2017) Medical laboratory associated errors: the 33-month experience of an on-line volunteer Canadian province wide error reporting system. Diagnosis (aop); (2) Carraro, P. (2007) Errors in a Stat Laboratory: Types and Frequencies 10 Years Later. Clin. Chem. 53(7) (3) Plebani, M. (2006) Errors in clinical laboratories or errors in laboratory medicine? Clin. Chem. Lab. Med. 44 (6)750-759




