BIOTIN SUPPLEMENTATION AND LABORATORY INTERFERENCE: An Unintended Consequence in the Pursuit of Beauty
Biotin is non-toxic, even at large doses, and excess quantities are cleared by the kidneys. Although high levels of biotin are not harmful physiologically, they may compromise the results of laboratory tests which employ biotin-streptavidin technology. Biotin-streptavidin binding is commonly found in immunoassays due to its avidity, sensitivity, specificity, and stability. A broad range of tests, such as those used in the diagnosis and/or monitoring of cardiac disease, endocrinopathies, malignancies, anemias, autoimmune and infectious diseases, may be affected by biotin.
Vitamin B7, more commonly known as biotin, is a water-soluble vitamin that functions as a coenzyme for carboxylase reactions. With a recommended dietary reference intake (DRI) of 30 μg/day, biotin supplementation is seldom indicated in healthy individuals, as biotin is found in various foods, including eggs, fish, meat, cauliflower, spinach, and avocado. Biotin in daily doses of 5-30 mg may be prescribed in certain inborn errors of metabolism, such as biotinidase deficiency or propionic acidemia.
More recently, megadoses up to 300 mg/day have shown promise in research studies to improve symptoms in patients with secondary progressive multiple sclerosis [1].

In the past several years, biotin supplementation has exploded in popularity as a beauty tool marketed to enhance hair, skin, and nail growth, although limited evidence exists to support such claims. A survey conducted in our outpatient laboratory at Vancouver General Hospital found up to 10% of the local population to be on biotin supplementation.
Biotin is non-toxic, even at large doses, and excess quantities are cleared by the kidneys. Although high levels of biotin are not harmful physiologically, they may compromise the results of laboratory tests which employ biotin-streptavidin technology. Biotin-streptavidin binding is commonly found in immunoassays due to its avidity, sensitivity, specificity, and stability. A broad range of tests, such as those used in the diagnosis and/or monitoring of cardiac disease, endocrinopathies, malignancies, anemias, autoimmune and infectious diseases, may be affected by biotin.
Much variability exists in the magnitude and direction of interference: in competitive immunoassays (e.g. for measurement of small molecules, such as free thyroxine [FT4] or free triiodothyronine [FT3]), the assay signal is inversely proportional to the analyte concentration. Biotin reduces the signal and leads to spuriously high results (Figure 1A), which might be inadvertently interpreted as an indication of hyperthyroidism.
In non-competitive, or sandwich, immunoassays (e.g. for measurement of larger molecules, such as thyroid-stimulating hormone [TSH] or cardiac troponin), supplemental biotin competes with biotinylated complex for binding to regent streptavidin-coated beads, decreasing the assay signal and causing factitiously low results (Figure 1B), which would also be consistent with a false indicator of hyperthyroidism. A number of cases have surfaced in the medical literature describing apparent biochemical thyrotoxicosis/Graves’ disease from biotin use [2,3].
The US Food and Drug Administration (FDA) has also issued an alert following an increase in reported adverse events from biotin interference with laboratory tests, including one death following a falsely low troponin result (troponin is released when the heart muscle has been damaged, such as a heart attack. The more damage there is to the heart, the greater the amount of troponin there will be in the blood).
Given the potential for significant patient harm, it is vital that hea lth care providers and patients be well-informed on the effect of biotin on laboratory tests. As part of history-taking, physicians should regularly inquire whether a patient is taking over-the-counter supplements, including biotin, and to advise that the patient refrain from biotin use for at least 1 day before a blood test is performed, or up to a week if taking very high doses. The half-life of biotin depends on a number of factors, including the dose, the duration of biotin use, and the patient’s kidney function. For a single dose of 600 μg, the half-life of biotin has been reported as <2 hours in individuals with normal renal parameters. In contrast, the half-life varies between 8 and 19 hours when a single dose between 100 mg and 300 mg is ingested [4].
lth care providers and patients be well-informed on the effect of biotin on laboratory tests. As part of history-taking, physicians should regularly inquire whether a patient is taking over-the-counter supplements, including biotin, and to advise that the patient refrain from biotin use for at least 1 day before a blood test is performed, or up to a week if taking very high doses. The half-life of biotin depends on a number of factors, including the dose, the duration of biotin use, and the patient’s kidney function. For a single dose of 600 μg, the half-life of biotin has been reported as <2 hours in individuals with normal renal parameters. In contrast, the half-life varies between 8 and 19 hours when a single dose between 100 mg and 300 mg is ingested [4].
In patients with recent biotin consumption who need bloodwork urgently – such as those presenting to the emergency department – the laboratory should be notified so that testing with alternative methods (i.e. those free of biotin-streptavidin technology) may be arranged. Although biotin can theoretically be removed from a sample via pre-treatment with streptavidin-coated microparticles [5], this is an expensive, labor-intensive option, with limited availability, and will necessitate prior procedural validation to ensure the extra step does not alter the method.
Laboratory professionals should be proactive in seeking out information from instrument manufacturers and the medical literature to ascertain the vulnerability of each assay to biotin use, and the threshold for interference when applicable. If a patient’s laboratory results are inconsistent with the clinical presentation, the laboratorian should be consulted to advise on the likelihood of biotin interference, and to organize follow-up investigations. With proper education and open communication between laboratory and clinical disciplines, risks from biotin interference can be effectively mitigated.
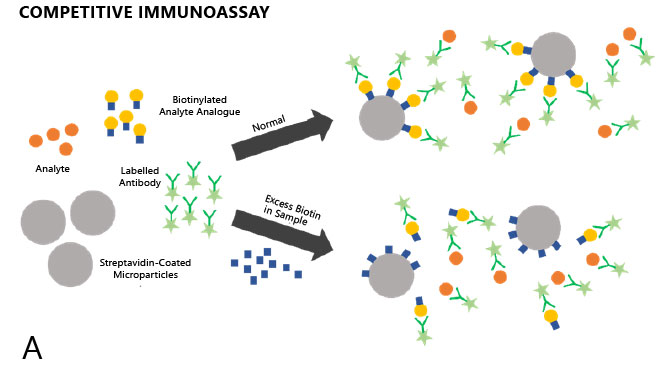
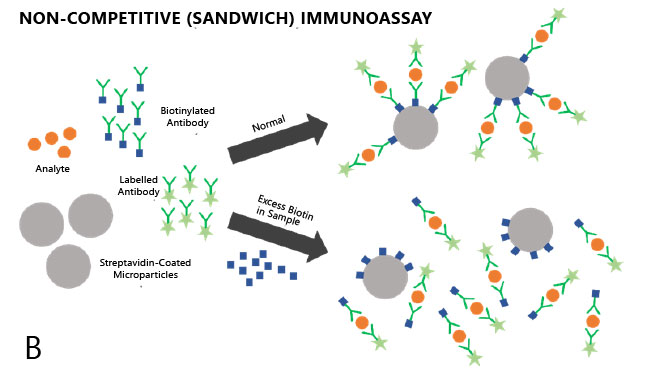
Figure 1. Competitive (A) and non-competitive (B) immunoassays in the presence and absence of excess biotin.
References
1. Tourbah A, Lebrun-Frenay C, Edan G, et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomised, double-blind, placebo-controlled study. Mult Scler 2016;22:1719-1731.
2. Elston MS, Sehgal S, Du Toit S, et al. Factitious Graves’ disease due to biotin immunoassay interference: a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251-3255.
3. Kummer S, Hermsen D, Distelmaier F. Biotin treatment mimicking Graves’ disease. N Engl J Med 2016;375:704-706.
4. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and non-hormone assays in healthy adults. JAMA 2017;318:1150-1160.
5. Trambas C, Lu Z, Yen T, et al. Depletion of biotin using streptavidin-coated microparticles: a validated solution to the problem of biotin interference in streptavidin-biotin immunoassays. Ann Clin Biochem 2018;55:216-226.




